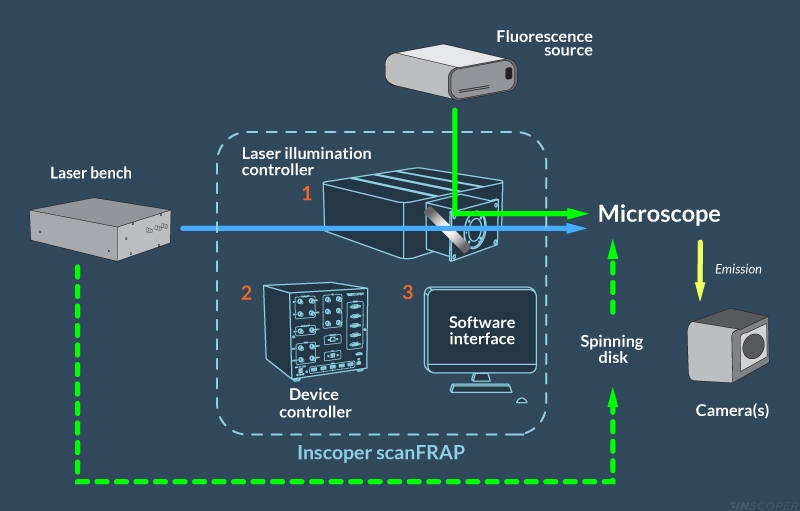
Inscoper scanFRAP is a turnkey solution for FRAP and photomanipulation applications, compatible with all brands of microscopes. It consists of (1) an optical module for controlling the laser illumination, (2) an electronic unit for triggering and synchronizing the microscope signals, and (3) complete image acquisition and processing software for operating all devices and functions of the microscope.
for live cell imaging
The Inscoper scanFRAP is developed for photomanipulation and optogenetics experiments in microscopy. Based on galvanometric mirror and optimized control technologies, this multi-purpose solution offers users capabilities for a wide range of in vitro, ex vivo and in vivo applications and can be combined within multidimensional acquisition sequences in the same software environment.

USER CASE @ BERKELEY UNIVERSITY
Upgrade of a TIRF microscope with Inscoper scanFRAP
Photomanipulation for biology
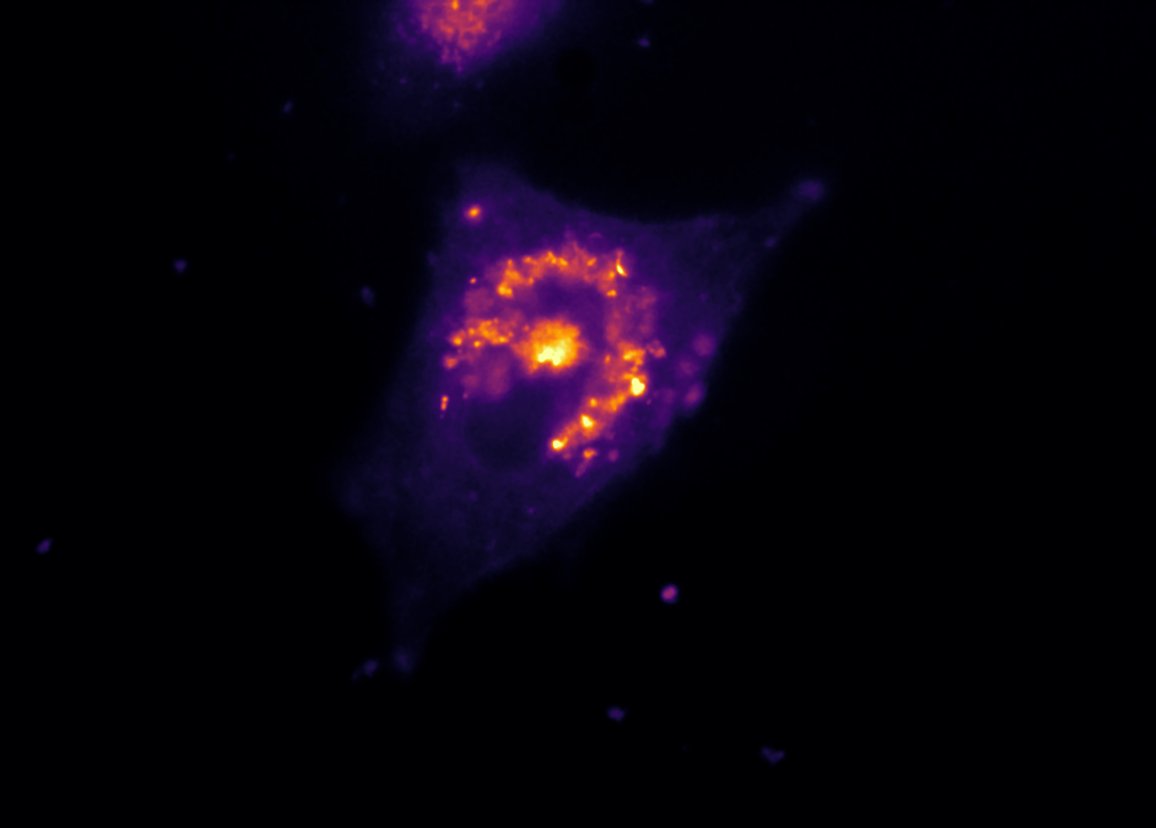
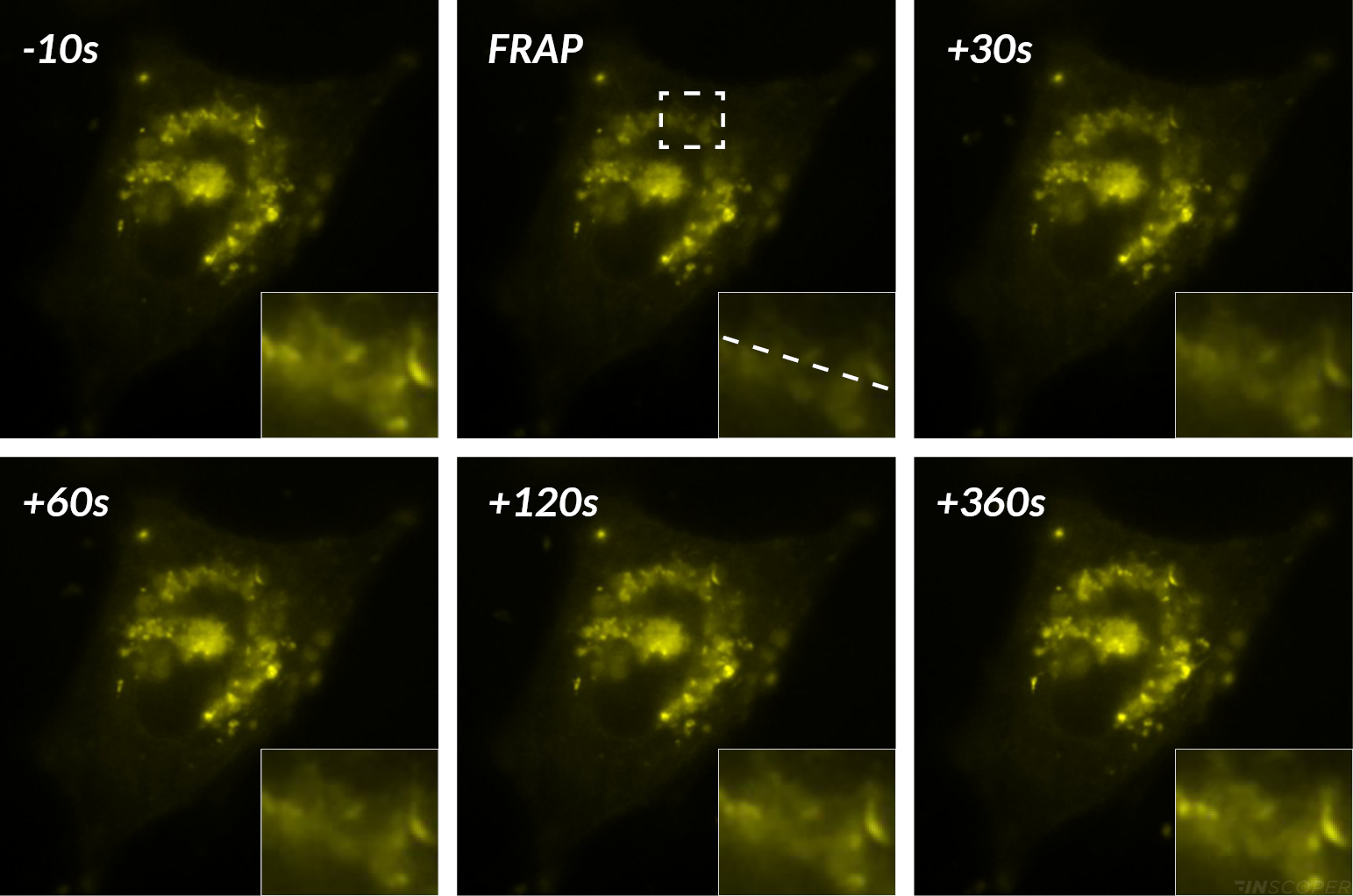
In microscopy, photomanipulation refers to a wide spectrum of techniques that allow biologists to easily interact with their living sample using a selected and specific illumination. These laser modulation techniques enable users to initiate/ modulate biological processes, to induce localized stress or to partially degrade a part of the specimen. One of the most common applications of photomanipulation in biology is Fluorescence Recovery After Photobleaching (FRAP).
It enables the characterization of the diffusion process of fluorescent-labeled organelles or proteins. A selected part of a cell is selectively photobleached, then the recovery time of the fluorescence is measured.
FRAP of HeLa cells expression YFP-RE
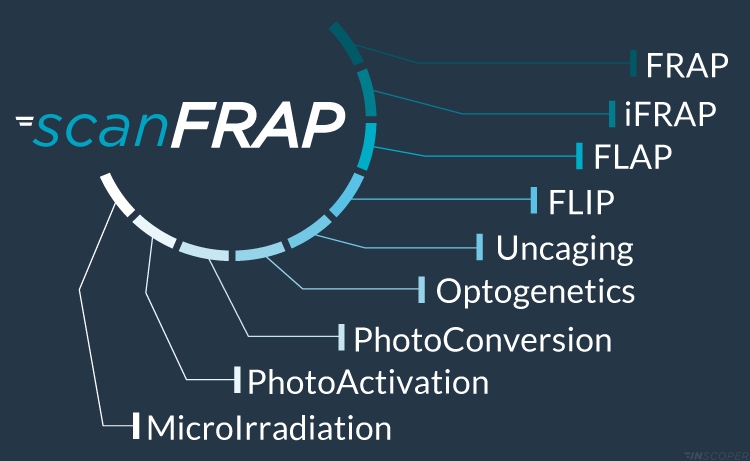
A large panel of biological applications
The Inscoper scanFRAP is a multi-application solution developed for high-performance photomanipulation and optogenetic experiments in microscopy. With hardware based on galvanometric mirror technology, the Inscoper scanFRAP offers users a large range of in vitro, ex vivo and in vivo applications such as iFRAP (inverse FRAP), FLIP (Fluorescence Loss In Photobleaching), FLAP (Fluorescence Losalization After Photobleaching), photoactivation, photoconversion, uncaging, microirradiation and optogenetics.
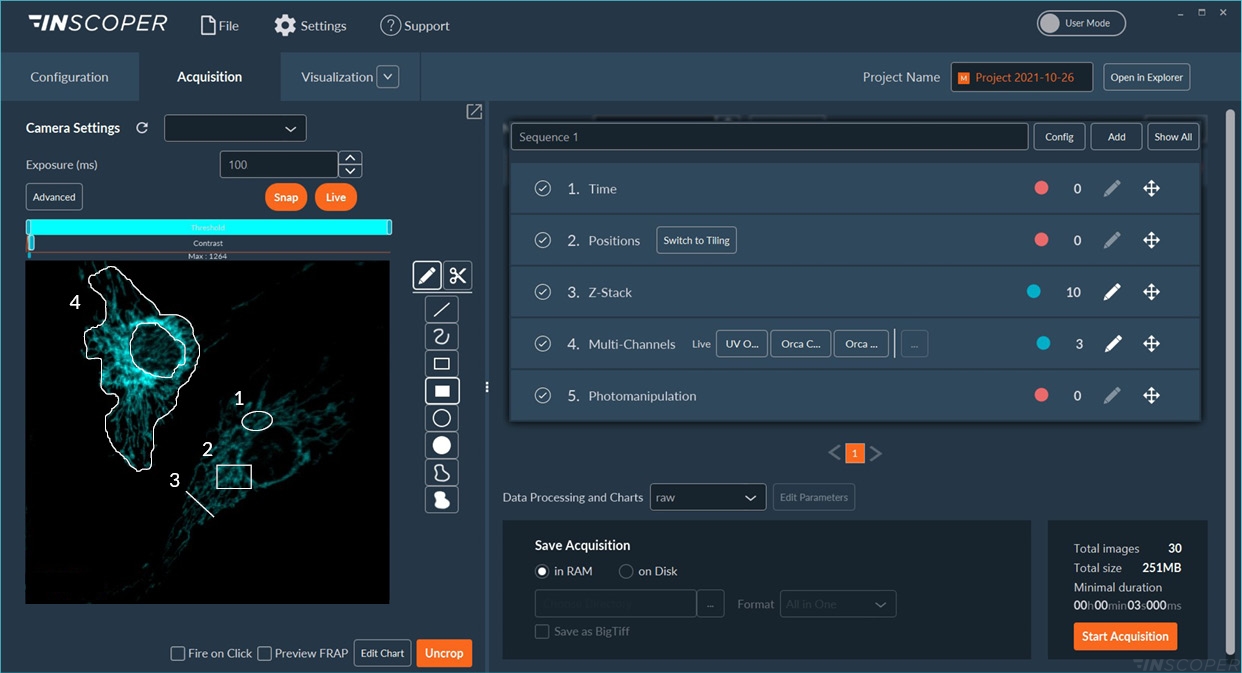
intuitive software environment for all microscope users
The graphical user interface of Inscoper software has been designed to address all kinds of needs in microscopy acquisition, with the aim to always keep a simple and intuitive user experience. The interface has only one window, with 3 tabs corresponding to the 3 steps of the acquisition: Configuration, Acquisition, Visualization. There are no complicated drop-down menus, though all the features for advanced microscopy are there.
ALC1 recruitment to DNA damages following laser micro-irradiation in living cells
A customizable solution to suit with your experimental design
With the same user-friendly interface as the Inscoper Imaging Solution, biologists can “draw” the region of interest (ROI) to bleach using conventional (line, circle, square) or custom-made (freehand drawing or new patterns) tools. The Inscoper scanFRAP also has a “Fire-on-Click” feature, that allows users to add/remove/modify ROI to photobleach in real time during acquisitions. Following an automated calibration, the Inscoper scanFRAP enables the use of customized photomanipulation protocol for each ROI (wavelength, pulse time, pulse iteration, laser diameter, laser power, dwell time).
INSCOPER technology for fast imaging in live cells
Users also benefit from the performance of the Inscoper Imaging Solution for optimized control, which guarantees the highest possible acquisition rate. Experiments with INSCOPER technologies strictly synchronize the illumination time with the exposure time, thus limiting the phototoxicity in living samples. It also enables the user to image the evolution of the fastest biological phenomena following photomanipulation (protein recruitment, genesis and dynamics of intracellular organelles, cell-cell interaction, …).
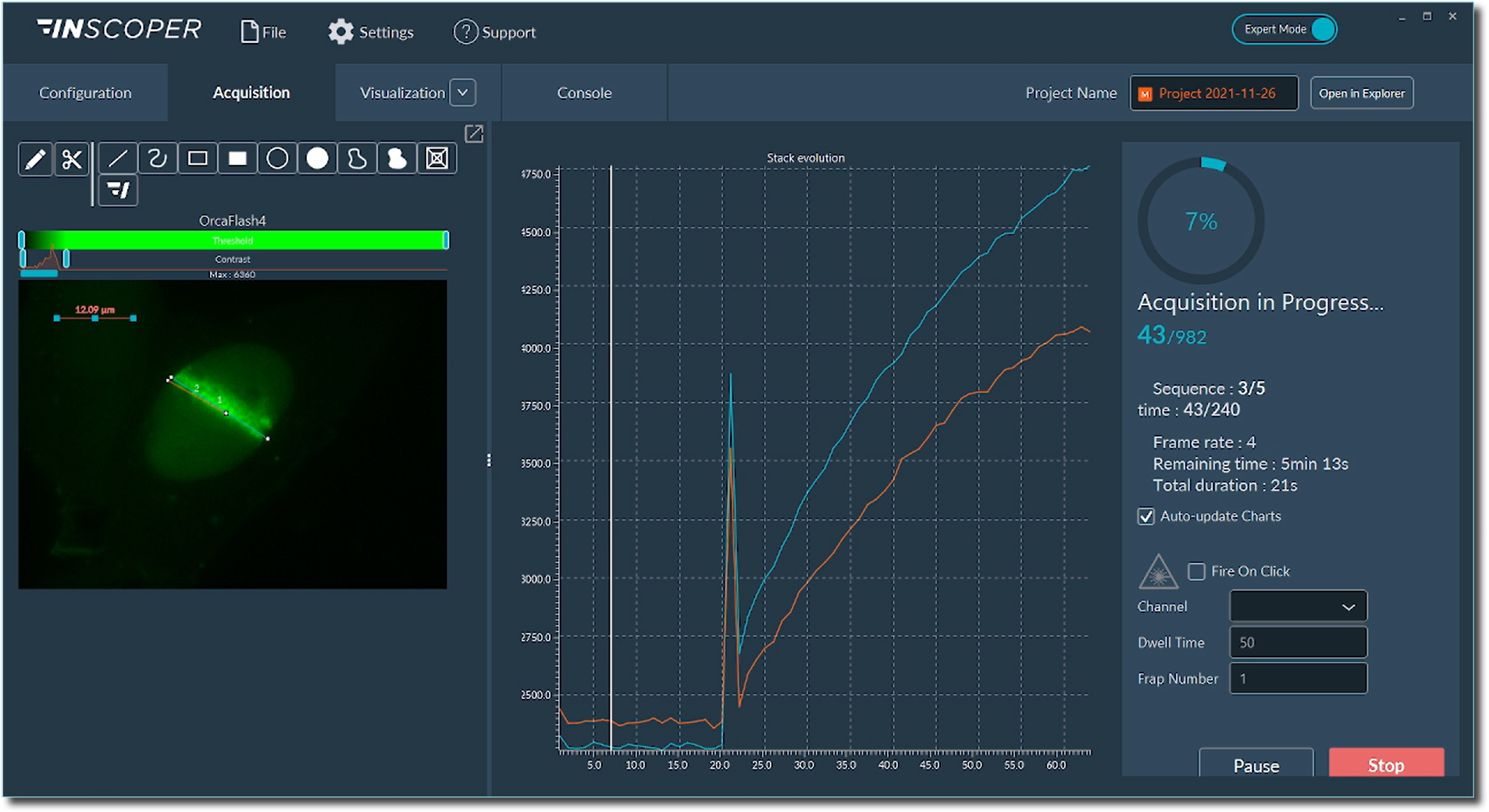
Live monitoring of fluorescence intensity modification
Live analyses are performed to monitor the evolution of the fluorescence intensity in all ROIs simultaneously. Each acquisition leads to the generation of .tif images and a .csv file containing all raw data of the fluorescence intensity changes for each ROI. A performance file is also generated to archive the acquisition sequence and the chain of commands. Such a file can be used for metrology applications for example.
The Inscoper scanFRAP is the perfect solution to perform photomanipulation experiments on living samples, combining a fully customizable illumination beam and a high spatial/temporal resolution.
RELATED APPLICATION NOTES
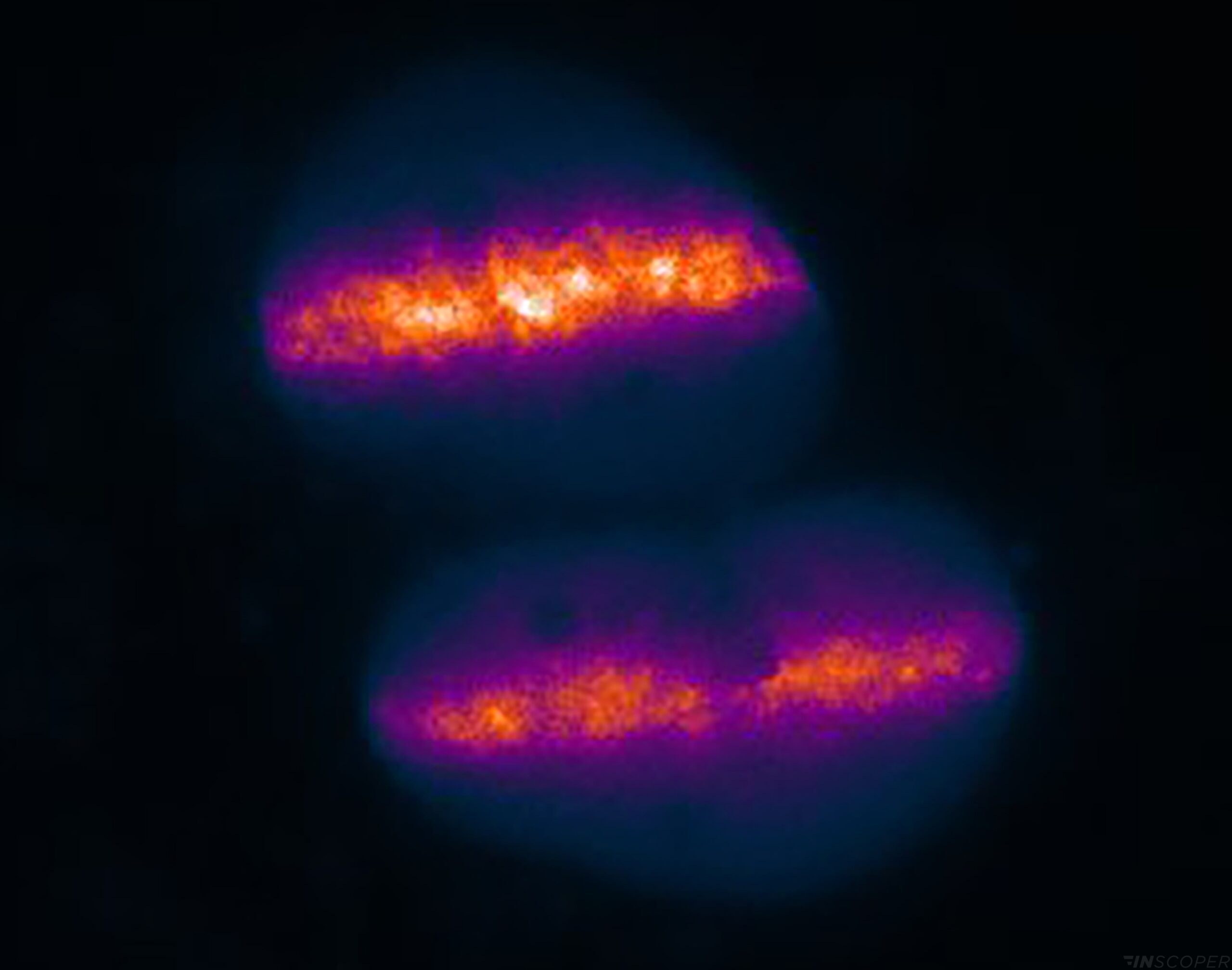
Microirradiation using Inscoper scanFRAP
Real-time DNA repair monitoring