Light-sheet fluorescence microscopy is an illumination technique well-suited for volumetric imaging that can be used for a wide spectrum of biological applications. Here, we present an opportunity for researchers to upgrade their macroscope to an “ultramacroscope”, a versatile instrument based on light-sheet illumination. This innovative technology was developed by Dr. Frédéric Brau from the MICA facility (Microscopy Imagerie Côte d’Azur) and Institute for Molecular and Cellular Pharmacology (IPMC) in collaboration with P. Girard and N. Mauclert from the Mechanical conception and realization workshop of the Observatoire de la Côte d’Azur (France) ; with the financial support of the GIS IBiSA (ITMO Cancer Plan Cancer 2009-2013). It is now integrated with the Inscoper Imaging Solution to optimize the acquisition sequences and facilitate their use by biologists.
ADVANTAGES OF LIGHT-SHEET FLUORESCENCE MICROSCOPY FOR 3D IMAGING
Tissues are composed of multiple polarized cell types organized in well-defined three-dimensional architecture. Imaging of large samples has become an essential technique to characterize biological processes (such as physiological or pathological development of tissues/organs) and anatomical structures (vascularisation, innervation, …). The main difficulties of large specimen imaging are the heterogeneity of refractive index and composition of tissues, that limit the spatial resolution and the imaging depth (Wang et al, 2014). However, a large variety of clearing techniques have been developed to circumvent these issues (Richardson & Lichtman, 2015).
Discovered in 1902, the use of planar illumination in microscopy was then combined with fluorescence microscopy for biological applications (Siedentopf & Zsigmondy, 1902; Voie et al, 1993). The laser beam laterally illuminates the sample with a thin sheet of illumination at the focal plane and emitted fluorescence is then collected perpendicularly by a camera.
Light-sheet fluorescence microscopy (LSFM) is nowadays the ideal technique for cleared sample imaging (Huisken et al, 2004). It has several advantages compared to conventional optical sectioning microscopy approaches. It is a camera-based system providing a better sensitivity for the detection of photons. This approach also provides a greater acquisition speed due to the absence of scanning, limiting phototoxicity. Thus, LSFM provides researchers with a powerful approach to image fluorescence (endogenous or immunolabeled) of a wide range of biological samples, from organoids to cleared organs or whole-body mice.
UPGRADE A MACROSCOPE TO AN ULTRAMACROSCOPE
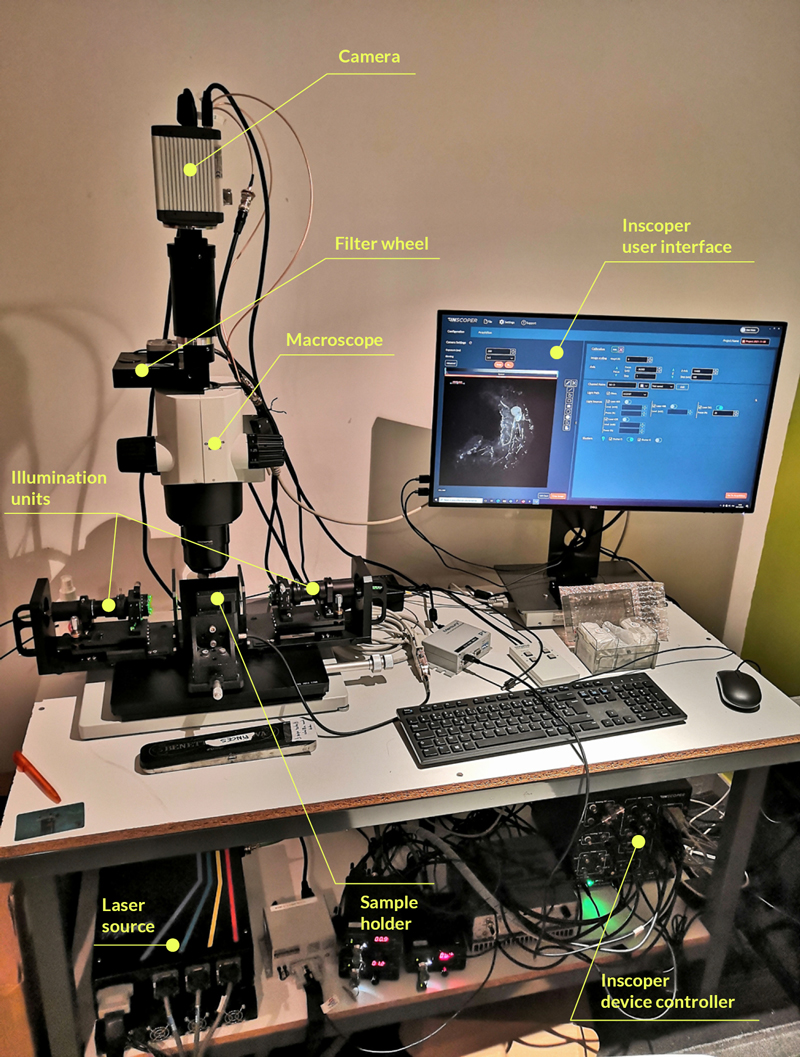
Frédéric Brau’s team (Institute for Molecular and Cellular Pharmacology [IPMC]; Nice, France) has recently developed an open and versatile light-sheet illumination module that can easily be implemented on commercial macroscopes (Leica, Nikon, Olympus, Zeiss). This system consists of a plug-and-play cylindrical-lens-based dual illumination unit (Figure 1). Here, the ultramacroscope used for this technical note was presented in a workshop during MiFoBio 2021 (Giens, France). It is based on a macroscope (MVX10; Olympus, Tokyo, Japan). Two illumination units are present and face each other, both of them are connected to the laser source (LBX-4C; Oxxius, Lannion, France) by an individual optical fiber. To prevent chromatic aberration, the beams pass through a double achromatic doublet (AC254-075-A-ML; Thorlabs, Newton, USA). The parallel beam is then projected onto a cylindrical doublet (ACY254-100-A, Thorlabs) through an iris diaphragm to induce the formation of two light sheets that are facing each other. Focalisation and co-alignment of the light sheets are done by the translation and the tilting of the illumination unit. A precise alignment of the sheets can be realized using a knife-edge prism (KRPB4-15-550; Optosigma Europe, Les Ulis, France). An orthogonal view of the 488nm and 561nm light sheets at different positions of the prism indicate the quality of the alignment. A sample holder is located at the intersection point of the beams and can be used to move the biological specimen vertically by a vertical translation stage (M-122.2DD; Physik Instrumente, Karlsruhe, Germany). The emitted fluorescent signal is collected through the 2X/0.5 objective, a filter wheel (Lambda 10-B; Sutter Instrument, Novato, CA, USA), and collected by an sCMOS camera (Flash4.0; Hamamatsu, Hamamatsu, Japan).
Figure 1: Ultramacroscope setup equipped with Inscoper Imaging Solution
This system was initially controlled with μManager software (http://www. micro-manager.org) and has been mentioned in several publications (Simon et al, 2017; Guyot et al, 2019a, 2019b). However, the lack of software ergonomics made the user experience tedious. The research team decided to change for the Inscoper Imaging Solution to have all devices integrated and controlled in a user-friendly environment. The images are generated in an open format compatible with the 3D visualization software and analysis workflow.
INSCOPER IMAGING SOLUTION FOR HOME-MADE IMAGING SYSTEMS
The Inscoper Imaging Solution is a full image acquisition solution for basic and advanced camera-based microscopes used in life science. It can be used on both commercial (Leica, Nikon, Olympus, and Zeiss) and home-made systems for the integration of a new device (camera, light source, spinning disk module, …) or a complete retrofit of the system. In all situations, the user-friendly graphical user interface allows the full control of all motorized devices, customizable calibration protocols, multidimensional acquisition (time, XYZ, channels, multi-positions, and tiling), and visualization. In addition to its universal and ergonomic aspect, the Inscoper technology improves the temporal resolution of acquisitions by removing all software latency and optimizing the synchronization of the elements of the microscope (Figure 2).
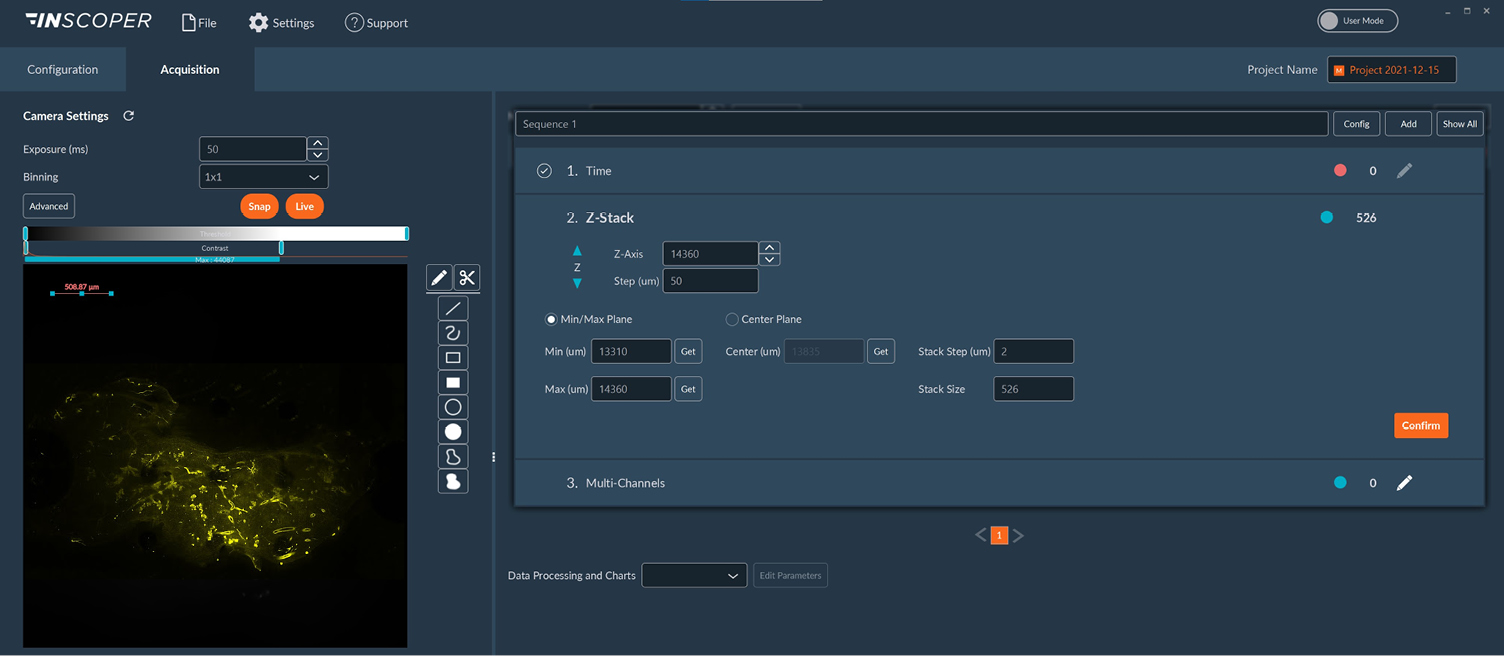
Figure 2: User interface of the Inscoper software
Overview of the Inscoper user interface used to manage the multidimensional acquisitions on the home-made ultramacroscope including timelapse, Z-stack, and multi-channels. All of these dimensions are fully customizable to be more suitable for user’s experiments. The software also provides users with a dedicated calibration protocol to perform the co-alignment of both light sheets before imaging.
BIOLOGICAL APPLICATIONS
The ultramacroscope, equipped with the Inscoper Imaging Solution, was shown during the microscopy event MiFoBio 2021 (Giens, France)during a workshop entitled “Immunolabeling and tissue clearing, acquisitionwith home-made versus commercial system” animated by Sophie Abélanet (Institute for Molecular and Cellular Pharmacology [IPMC]; Nice, France) and Dr. Chloé Dominici (Institute for Research on Cancer and Aging [IRCAN], Nice, France).
The system was used to image an entire mammary gland from a mouse (dimensions: 1.5×0.5cm). This organ was prepared using the iDISCO+ clearing protocol. This protocol was chosen for its compatibility with immunolabeling, its simplicity, and its low cost (Renier et al, 2016). It was also immunolabeled with a primary antibody against SMA (Smooth Muscle Actin) and a secondary antibody coupled with an Alexa 568. The SMA protein labels the myoepithelial cells in the mammary gland. These differentiated cells form the basal layer of mammary ducts, apposed to the basement membrane. Myoepithelial cells have different roles, including contraction to expel glandular secretions (Gieniec & Davis, 2022). The labeling of this protein is often used to characterize the structure of the whole tissue and evaluate the effect of the pathological phenomenon.
The ultramacroscope succeeded to image the whole organ (Figure 3). Then, images generated by the Inscoper Imaging Solution were opened with the open-source ImageJ software (https://imageJ.nih.gov/ij/) for processing and with the commercial Imaris software (Bitplane, Belfast, United Kingdom) for 3D visualization of the sample.
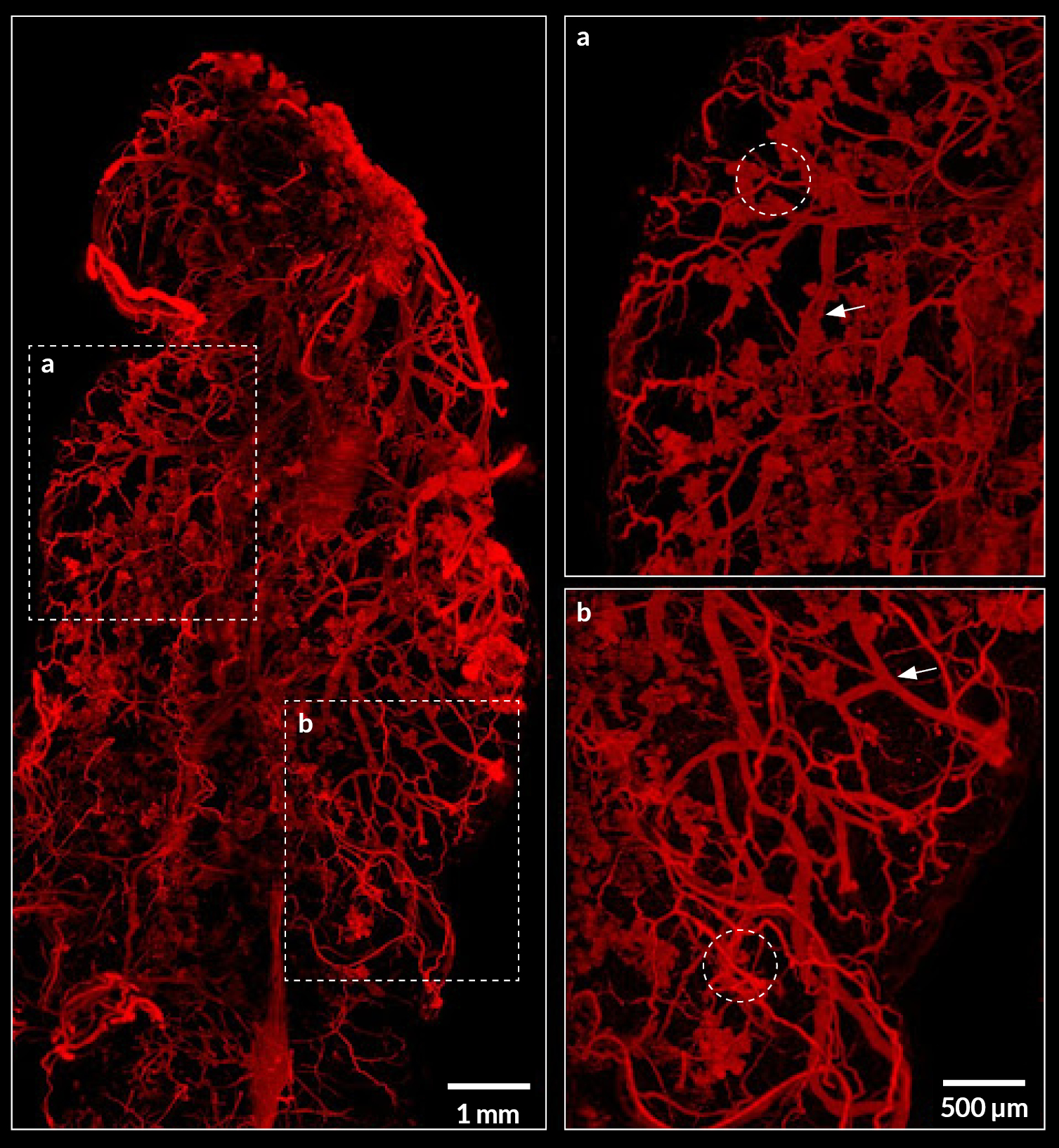
Figure 3: Imaging of myoepithelial cells in cleared mammary gland using the ultramacroscope
Maximum projection image of an entire iDISCO-cleared mammary gland with myoepithelial cells labeling (SMA, red). Two dashed rectangles are zoomed in at the right part of the image. Dashed circles represent some clusters of alveoli and arrows point to ducts. Scale bar: 1mm (left) and 500µm (right).
Summary
The home-made microscopy work presented in this technical note has enabled an innovative and cost-effective upgrade of a pre-existing system into an ultramacroscope. INSCOPER provided a tailor-made solution to improve the user experience and microscope control. The Inscoper Imaging Solution guarantees a perfect synchronization of all devices of the system, and a convenient imaging experience thanks to an intuitive and reliable software interface. Such a system can be used in a large spectrum of applications including oncology, development studies, the biodistribution of labeled molecules or characterization of biological structures (nervous system, blood, and/or lymphatic vessels, …).
Bibliography
- Gieniec KA & Davis FM (2022) Mammary basal cells: Stars of the show. Biochim Biophys Acta BBA – Mol Cell Res 1869: 1–6
- Guyot M, Simon T, Ceppo F, Panzolini C, Guyon A, Lavergne J, Murris E, Daoudlarian D, Brusini R, Zarif H, et al (2019a) Pancreatic nerve electrostimulation inhibits recent-onset autoimmune diabetes. Nat Biotechnol 37: 1446–1451
- Guyot M, Simon T, Panzolini C, Ceppo F, Daoudlarian D, Murris E, Macia E, Abélanet S, Sridhar A, Vervoordeldonk MJ, et al (2019b) Apical splenic nerve electrical stimulation discloses an anti-inflammatory pathway relying on adrenergic and nicotinic receptors in myeloid cells. Brain Behav Immun 80: 238–246
- Huisken J, Swoger J, Del Bene F, Wittbrodt J & Stelzer EHK (2004) Optical Sectioning Deep Inside Live Embryos by Selective Plane Illumination Microscopy. Science 305: 1007–1009
- Renier N, Adams EL, Kirst C, Wu Z, Azevedo R, Kohl J, Autry AE, Kadiri L, Umadevi Venkataraju K, Zhou Y, et al (2016) Mapping of Brain Activity by Automated Volume Analysis of Immediate Early Genes. Cell: 1789–1802
- Richardson DS & Lichtman JW (2015) Clarifying Tissue Clearing. Cell 162: 246–257
- Siedentopf H & Zsigmondy R (1902) Uber Sichtbarmachung und Größenbestimmung ultramikoskopischer Teilchen, mit besonderer Anwendung auf Goldrubingläser. Drudes Ann Phys: 1–39
- Simon T, Pogu J, Rémy S, Brau F, Pogu S, Maquigneau M, Fonteneau J-F, Poirier N, Vanhove B, Blancho G, et al (2017) Inhibition of effector antigen-specific T cells by intradermal administration of heme oxygenase-1 inducers. J Autoimmun 81: 44–55
- Voie AH, Burns DH & Spelman FA (1993) Orthogonal-plane fluorescence optical sectioning: Three-dimensional imaging of macroscopic biological specimens. J Microsc: 229–236
- Wang K, Milkie DE, Saxena A, Engerer P, Misgeld T, Bronner ME, Mumm J & Betzig E (2014) Rapid adaptive optical recovery of optimal resolution over large volumes. Nat Methods 11: 625–628
0 Comments